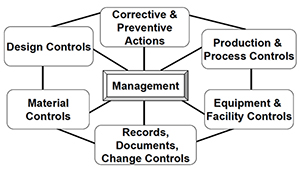
How to prove your SDLC is being followed for compliance with medical standards like IEC 62304 | by Laura at Kosli.com | Medium
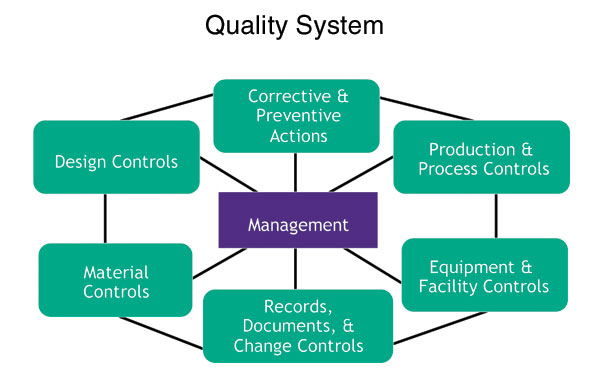
Medical device makers find solution to FDA demands - Tooling and Production Magazine for Large Plant Management and Metalworking
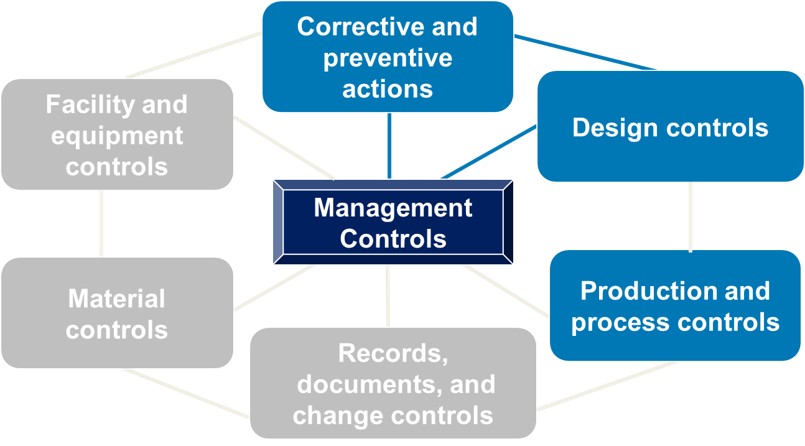


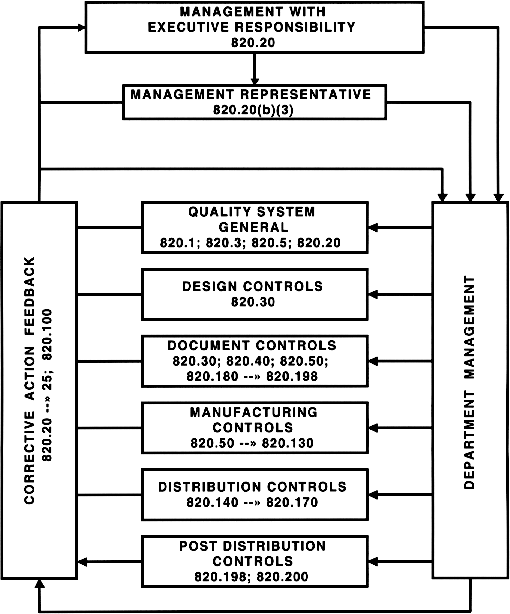

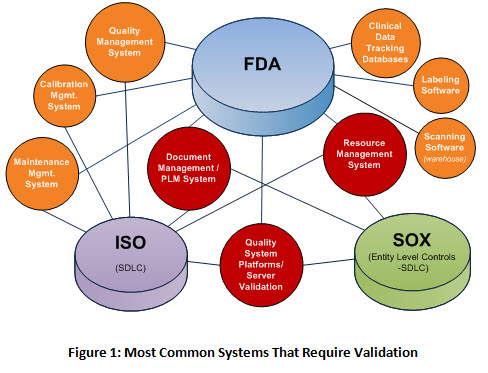

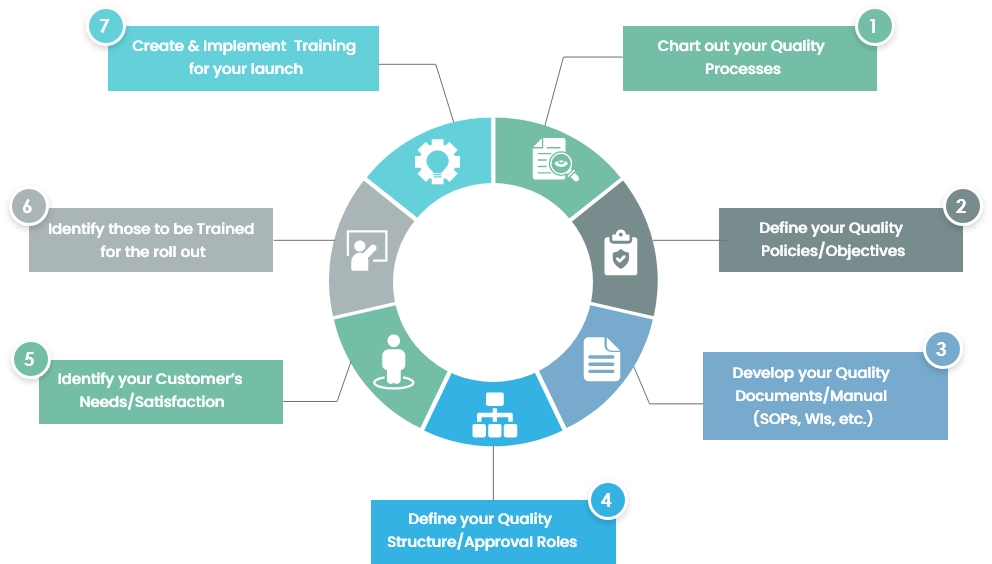
.webp?width=500&height=381&name=Quality%20management%20system%20product%20development%20(1).webp)