Donald S. Robinson, MD: Treatment Effects of Selegiline Transdermal System on Symptoms of Major Depressive Disorder: A Meta-Analysis of Short-Term, Placebo-Controlled, Efficacy Trials
These highlights do not include all the information needed to use EMSAM safely and effectively. See full prescribing information for EMSAM. EMSAM® (selegiline transdermal system) Initial U.S. Approval: 2006

Autism Capital 🧩 on X: "We've received confirmation that Sam's drug was actually Emsam. It is a drug normally used to treat Depression and Parkinson's disease but also gives performance enhancing and

Evidence that Formulations of the Selective MAO-B Inhibitor, Selegiline, which Bypass First-Pass Metabolism, also Inhibit MAO-A in the Human Brain | Neuropsychopharmacology

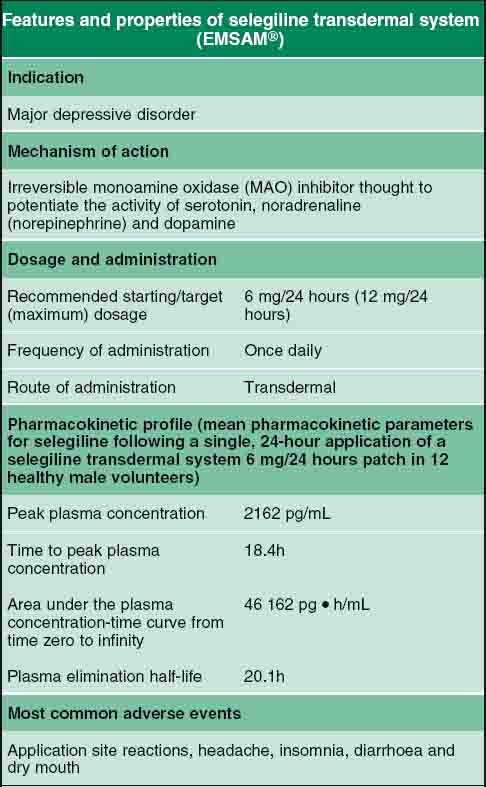
Table 3 from The selegiline transdermal system (emsam): a therapeutic option for the treatment of major depressive disorder. | Semantic Scholar

Table 3 from The selegiline transdermal system (emsam): a therapeutic option for the treatment of major depressive disorder. | Semantic Scholar